Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.

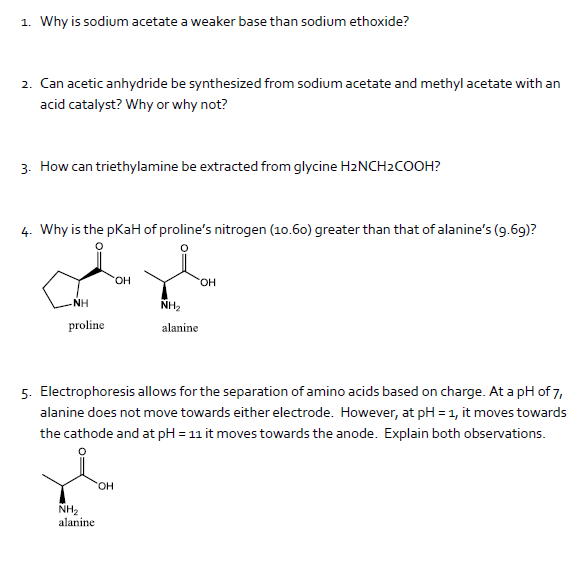
SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow

SOLVED: Acetic acid (CH3COOH, 𝐾a=1.80×10−5) is a weak acid, so the salt sodium acetate (CH3COONa) acts as a weak base. Calculate the pH of a 0.809 M solution of sodium acetate. pH=

Two buffer solutions, `A` and `B`, each made acetic acid and sodium acetate differ in their `pH`... - YouTube

In a mixture of acetic acid and sodium acetate, the ratio of concentrations of the salt to the acid is increased ten times. Then the pH of the solution:

What is the pH of buffer solution containing 0.17 M acetic acid and 0.36 M sodium acetate? - YouTube
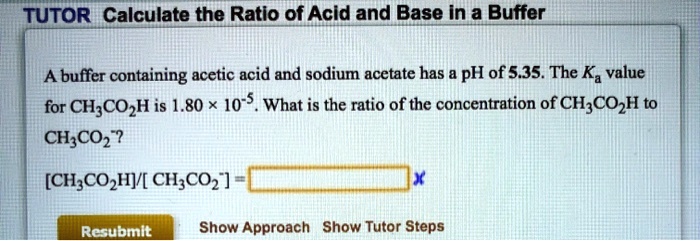
SOLVED: TUTOR Calculate the Ratio of Acid and Base In a Buffer A buffer containing acetic acid and sodium acetate has pH of 5.35. The Ka value for CH;COzH is 1.80 x
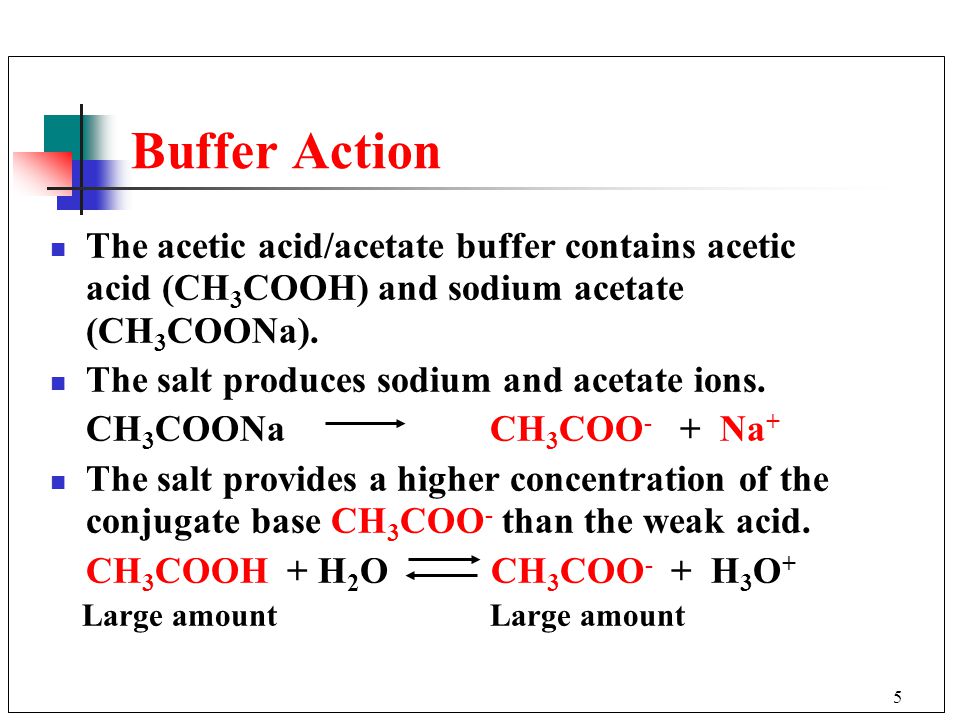
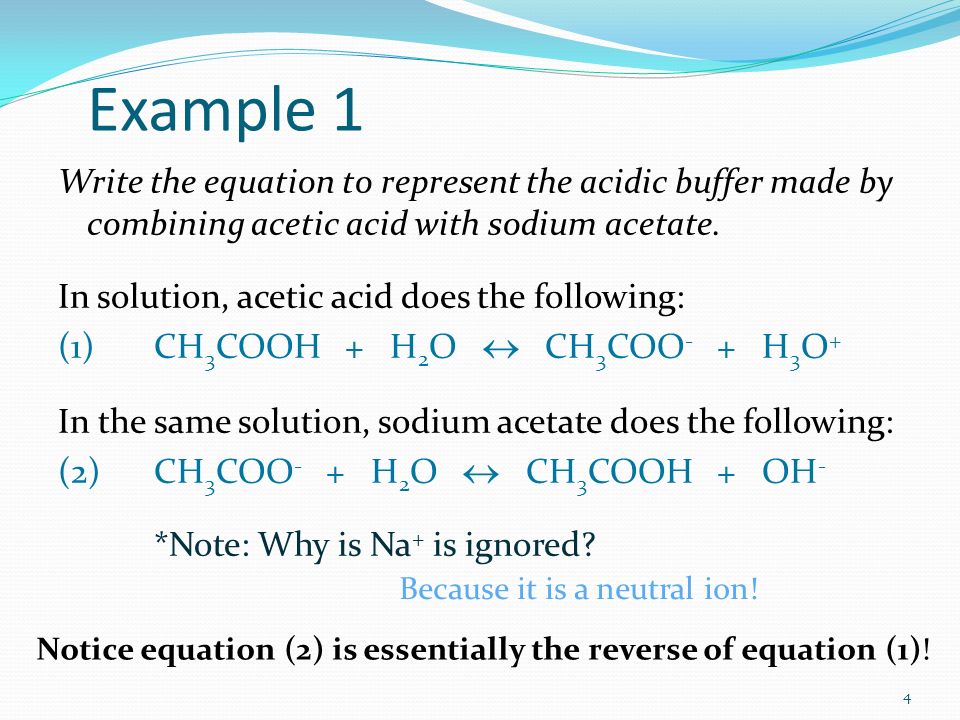
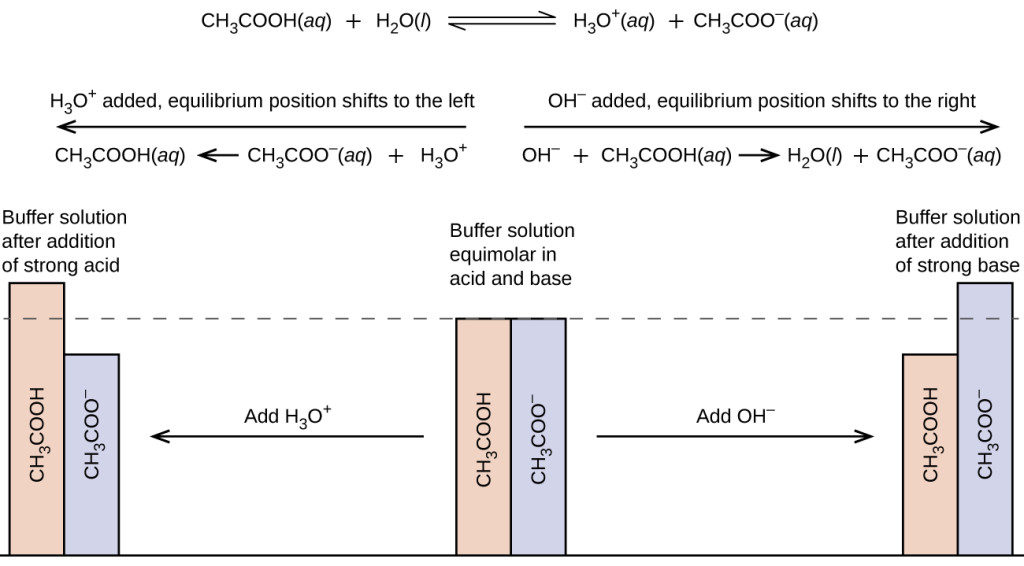
1 Chapter 10 Acids and Bases 10.9 Buffers. 2 When an acid or base is added to water, the pH changes drastically. A buffer solution resists a change in. - ppt download

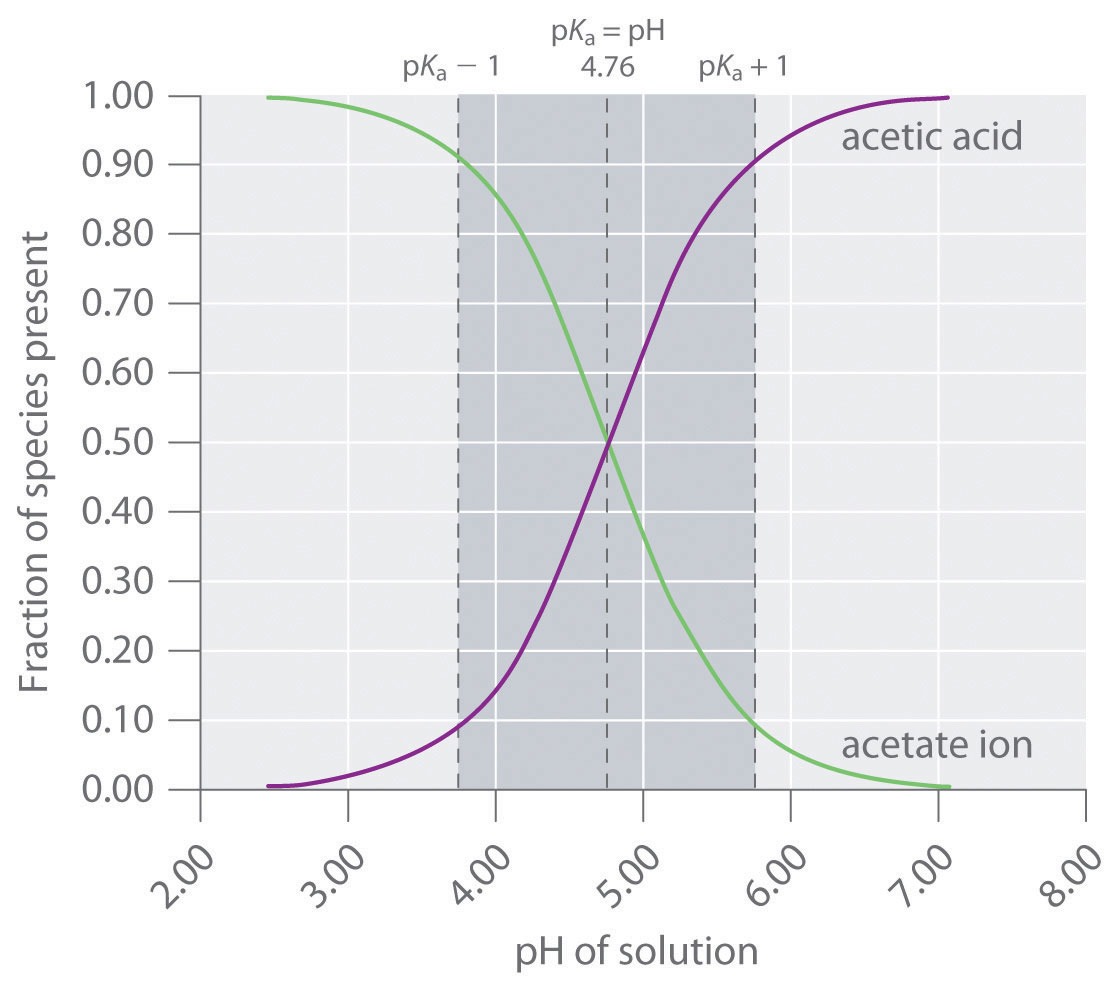
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid are mixed. The pKa of acetic acid is 4.76. The pH of the buffer solution is: