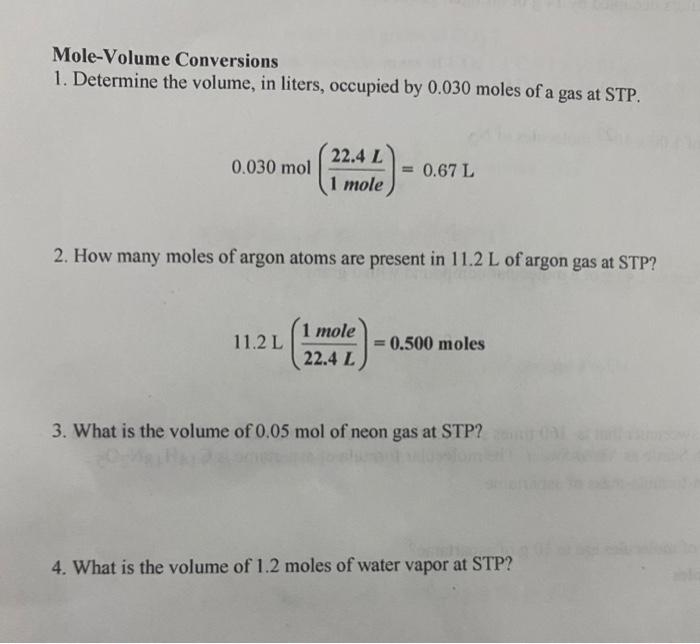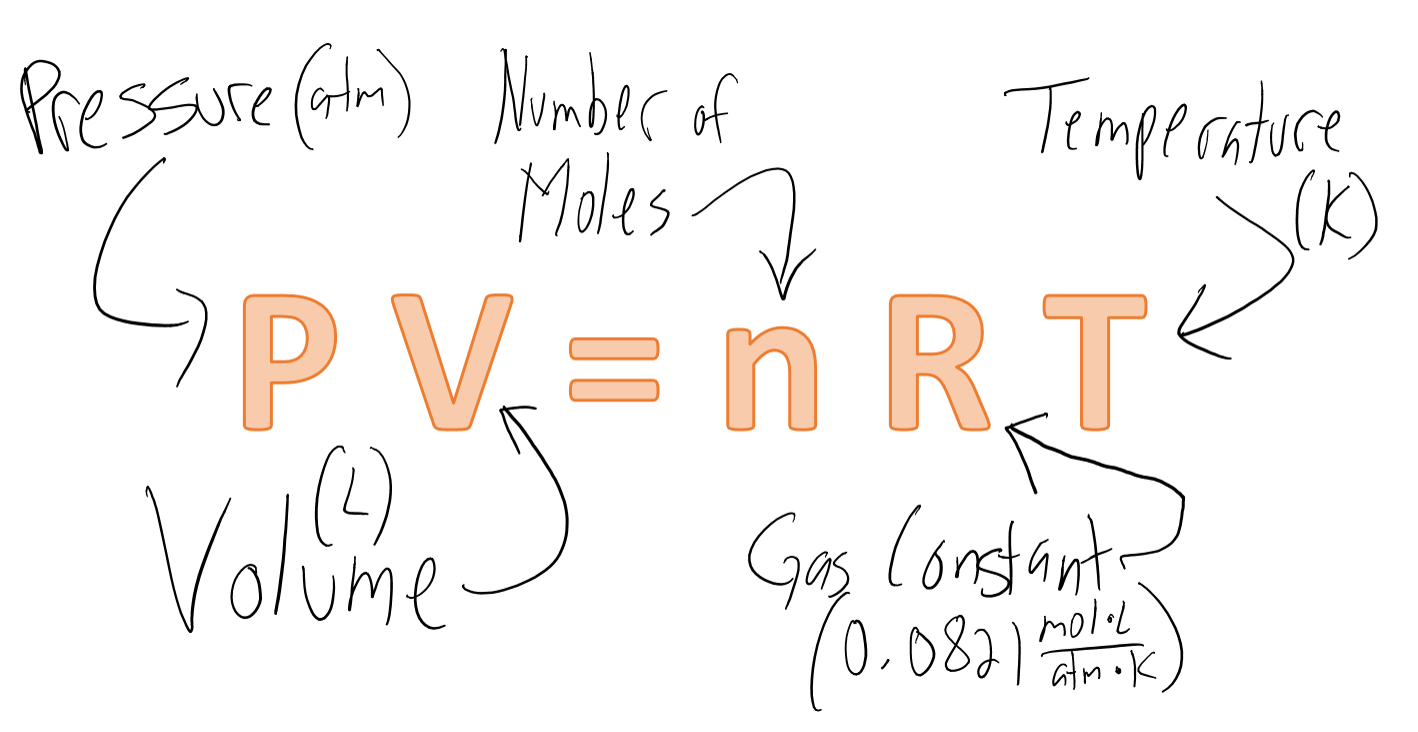Question 6 (1 point) Hydrogen gas and nitrogen gas can react to form ammonia according to this equation: N2 - Brainly.com

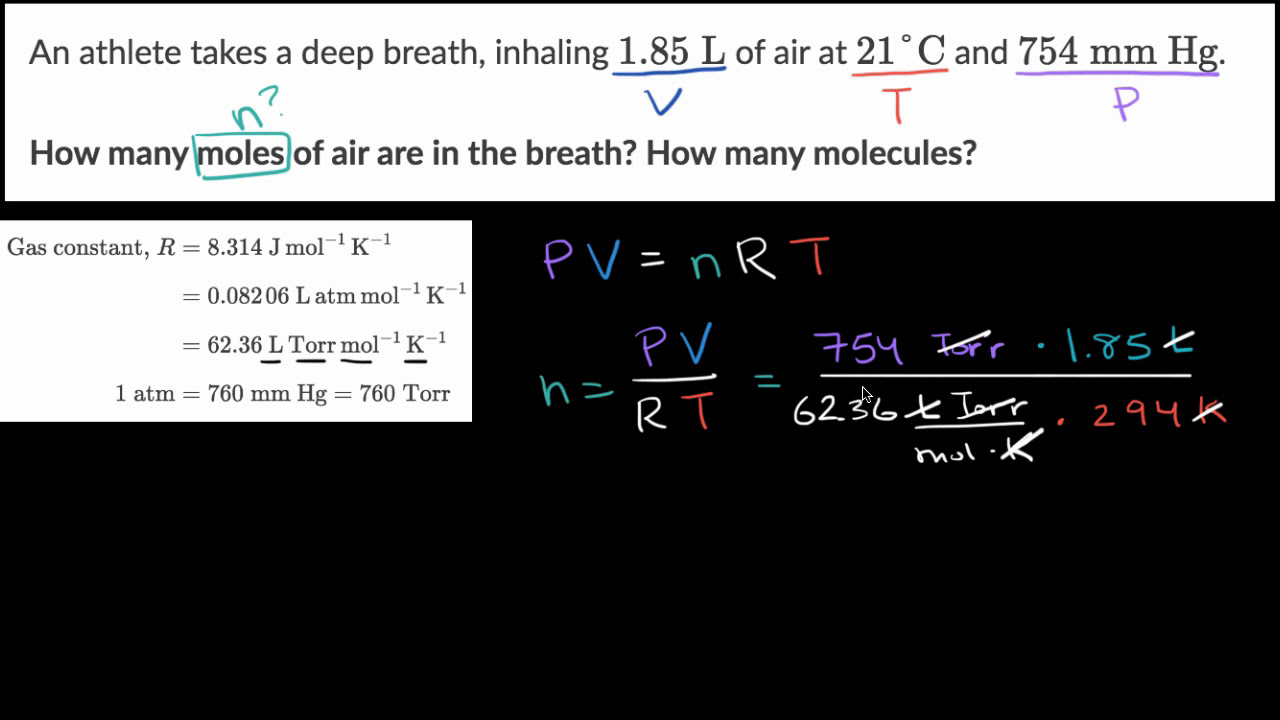
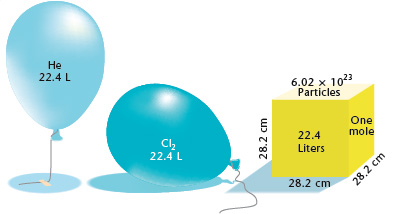
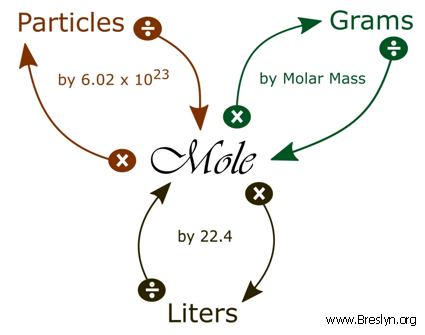
1 mole = 6.02 x 10^23 molecules. 1 mole= 22.4 L How many liters does 5.0 x 10^24 molecules occupy? .37 L - Brainly.com

Review: Mole Conversions: Convert 3 mols Oxygen to grams: Convert 42 grams Chlorine to mols: What is % composition? What is the %comp of magnesium in magnesium. - ppt video online download
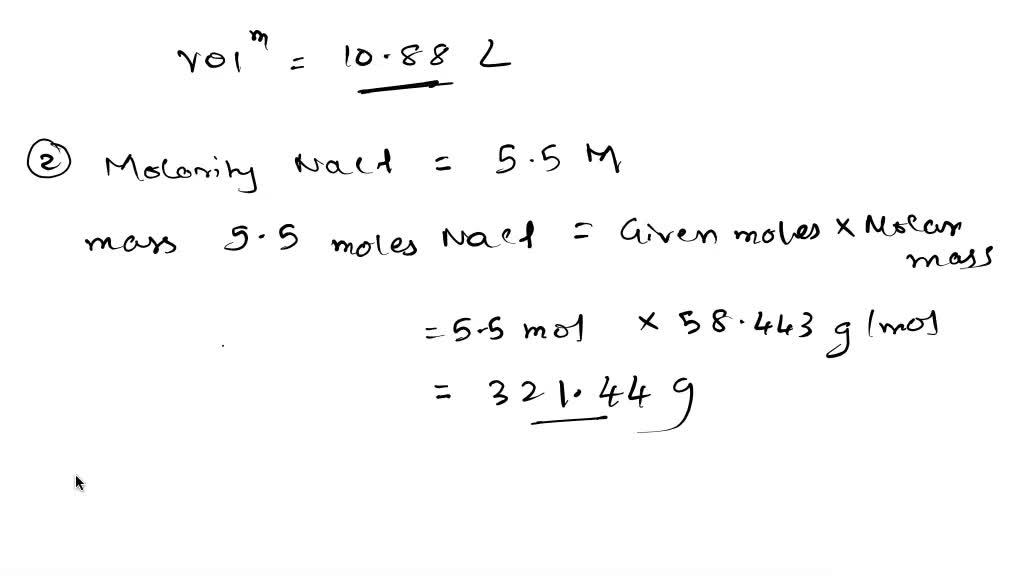
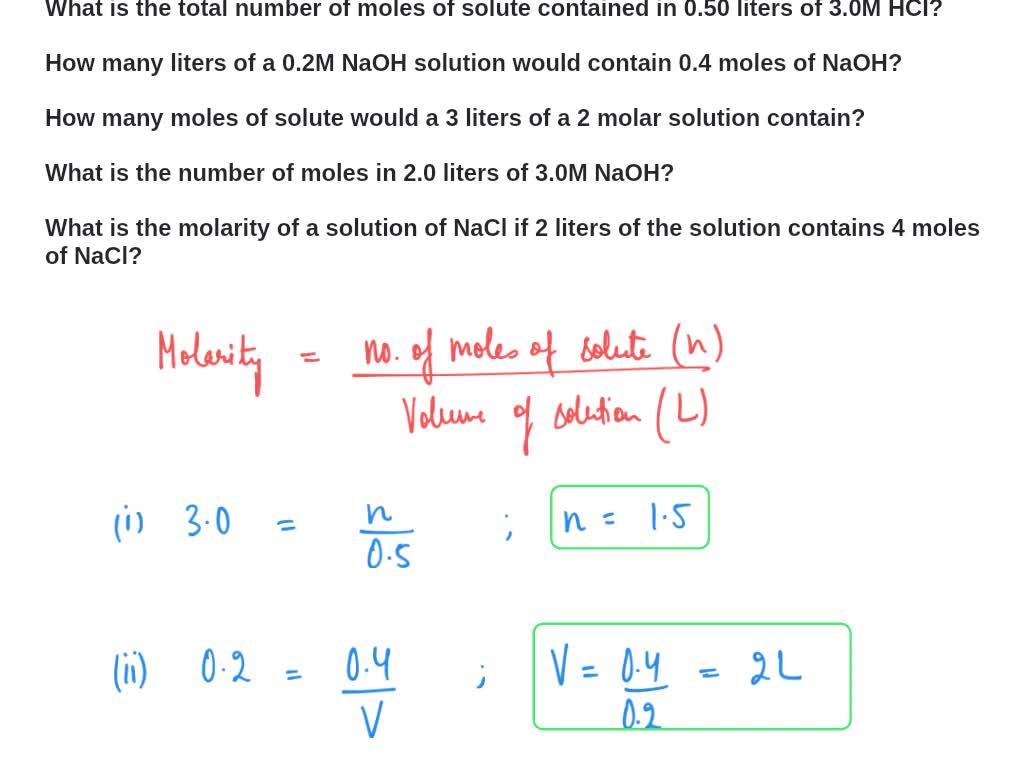
SOLVED: How many liters of a 0.114 M NaOH solution contains 1.24 moles of NaOH? The concentration of an NaCI solution (in water) expressed as Molarity is 5.5 M Express this concentration