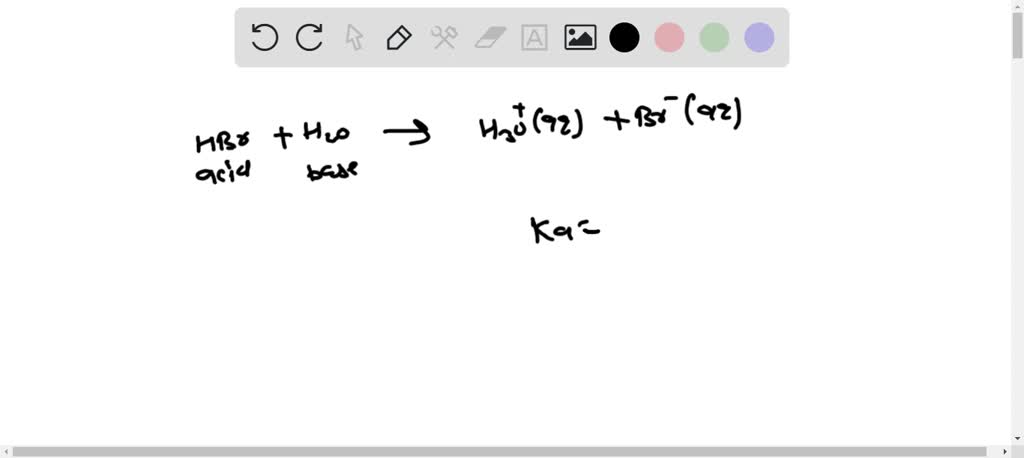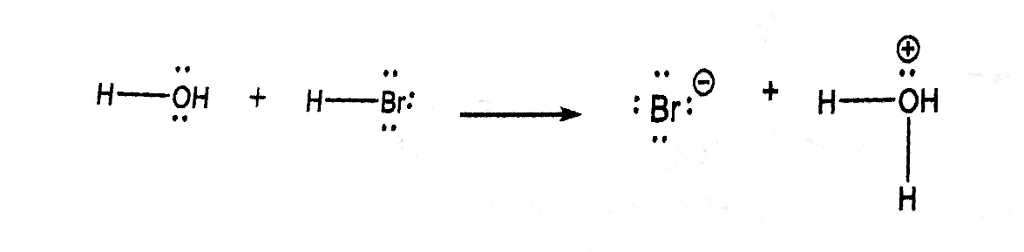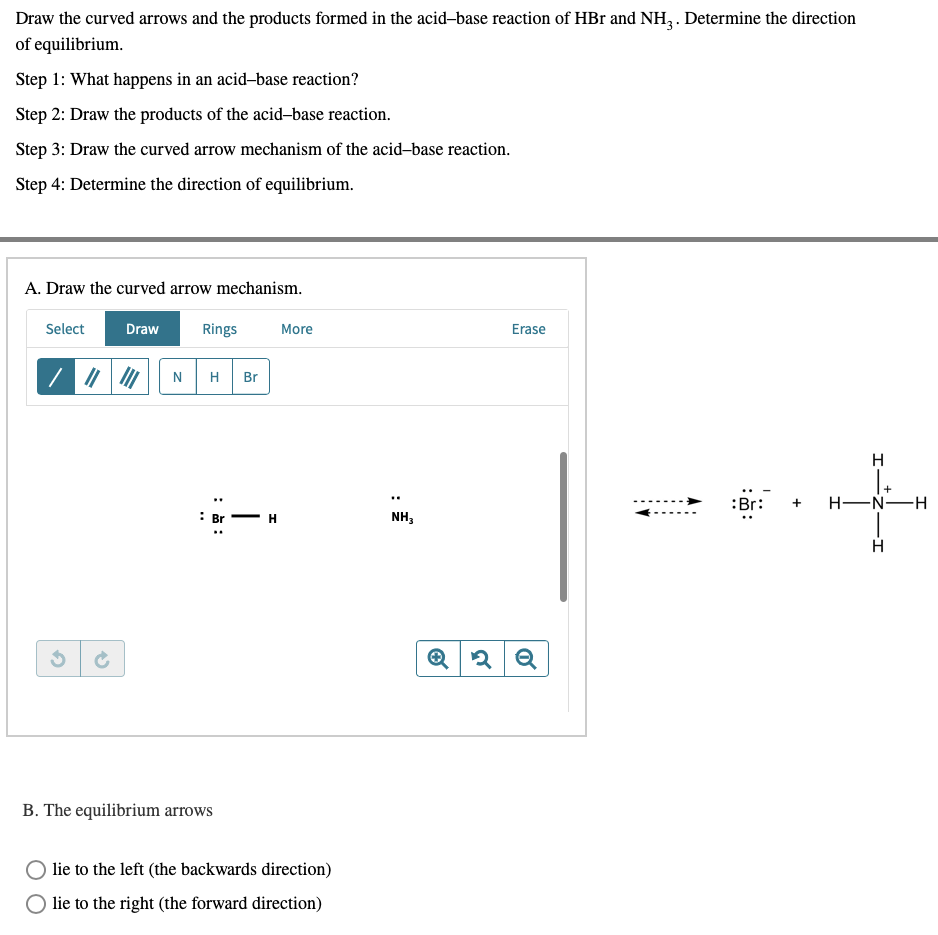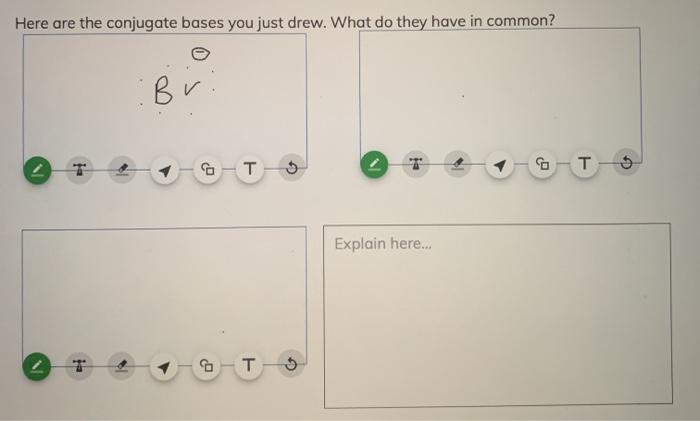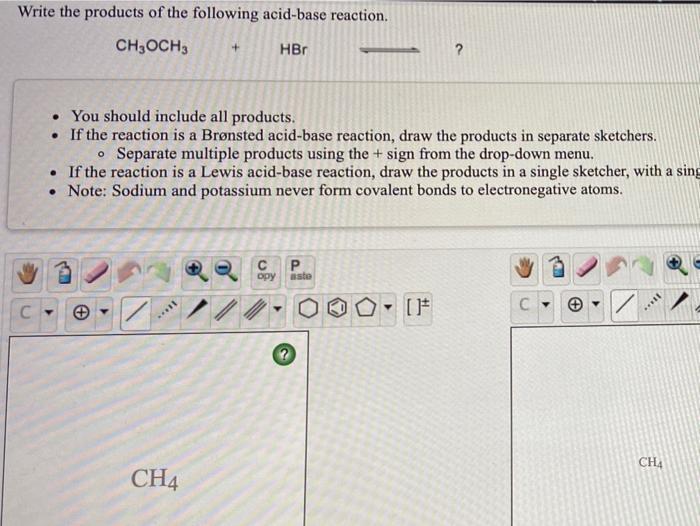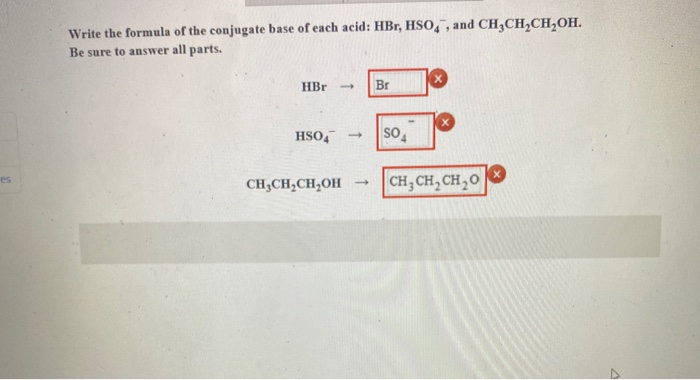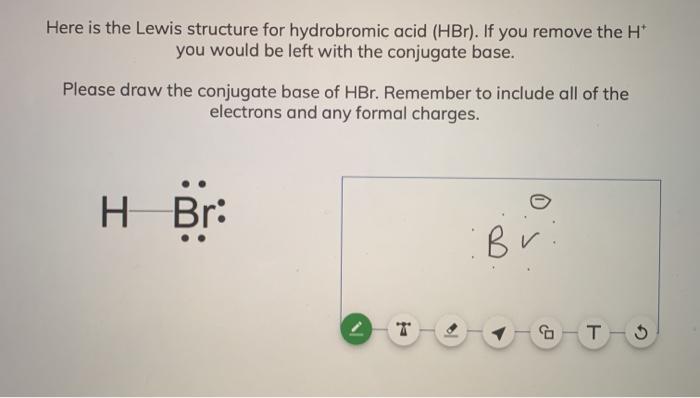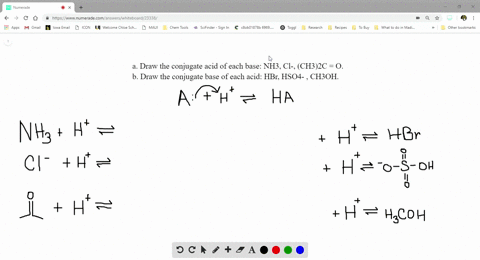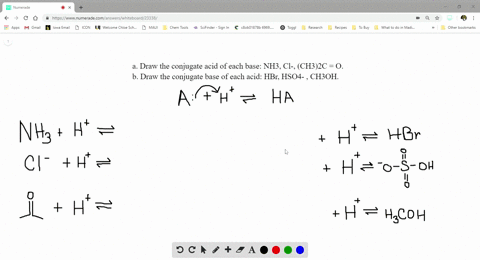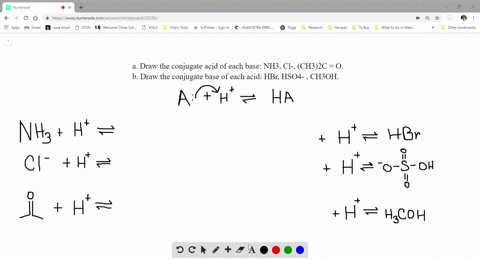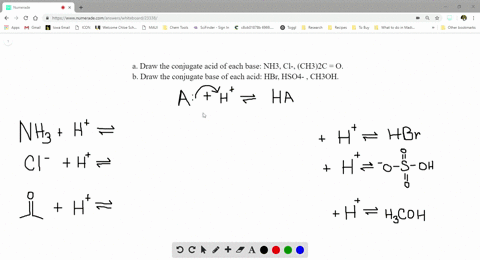
SOLVED:a. Draw the conjugate acid of each base: NH3, Cl^-, (CH3)2C = O. b. Draw the conjugate base of each acid: HBr, HSO4^- , CH3OH.

Existing literature and proposed circuits (a, b, c). HBR, DSHBR, HBRJT... | Download Scientific Diagram
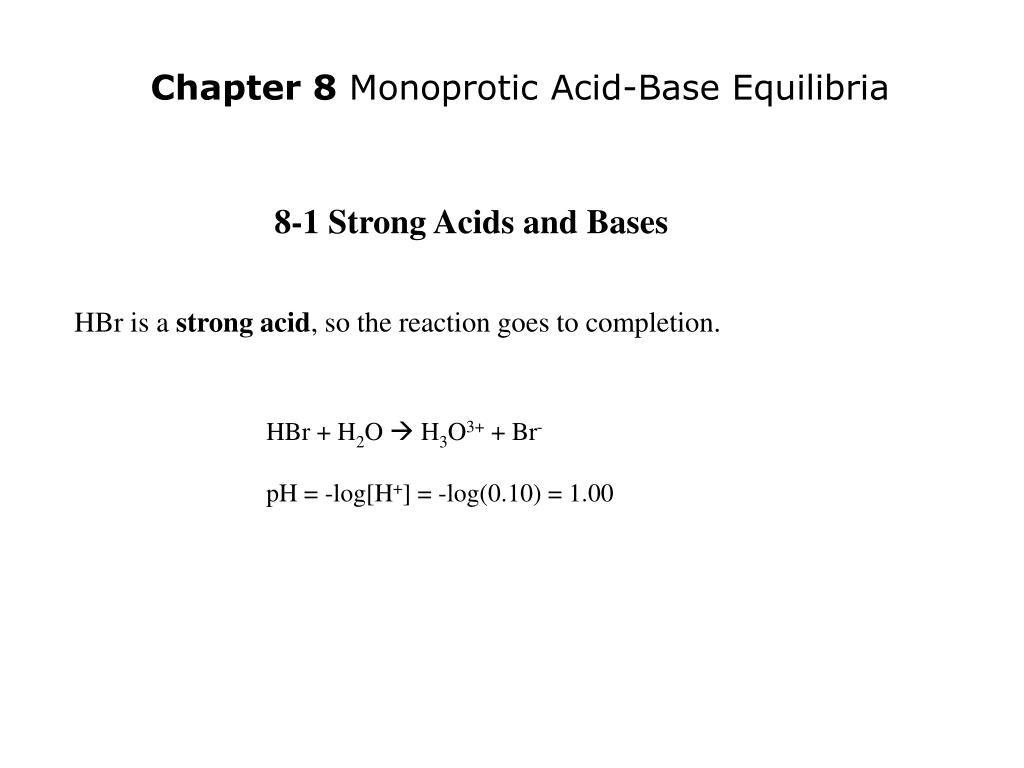
PPT - HBr is a strong acid , so the reaction goes to completion. PowerPoint Presentation - ID:4536389

HL Acids and Bases. Strength of Acids/Bases Strong Acids (100% ionized or dissociated) – HCl – HBr – HI – HNO 3 – H 2 SO 4 – HClO 4 – HClO 3 Strong bases. - ppt download
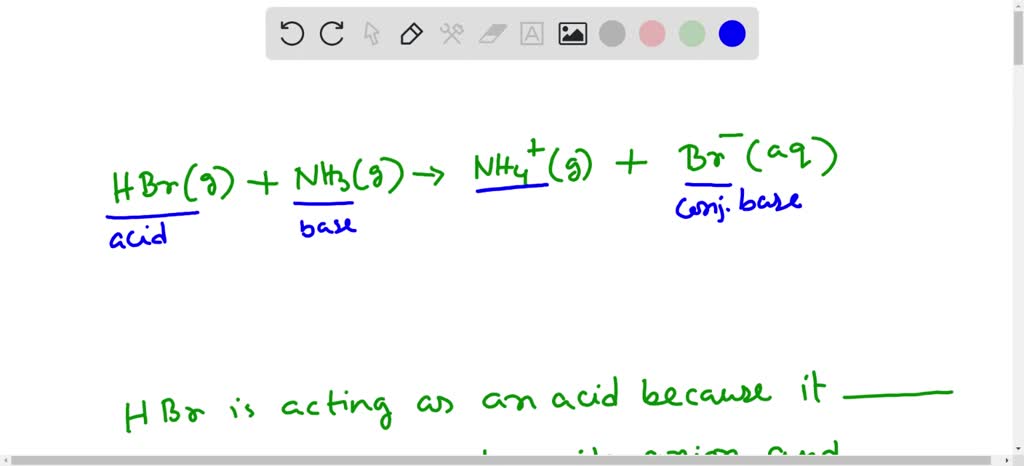
SOLVED: In the following acid-base reaction: HBr(g) + NH3(g) → NH4+(aq) + Br– (aq) HBr is acting as the acid, because it a proton to form bromide anion, and NH3 is acting
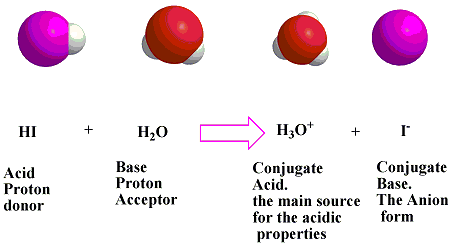
Which pair is a Brønsted–Lowry conjugate acid–base pair? NH_3 ; NH_4^+ or H_3O^+ ; OH^- or HCl; HBr or ClO_4^(-); ClO_3^- | Socratic

SOLVED: What is the conjugate base of HBr? Br+ B Br D: BrOH QUESTION 6 Which compound in the following pair is the stronger acid? CHBCHZCH3 (pKa = 50) and CHBCHZOH (pKa =