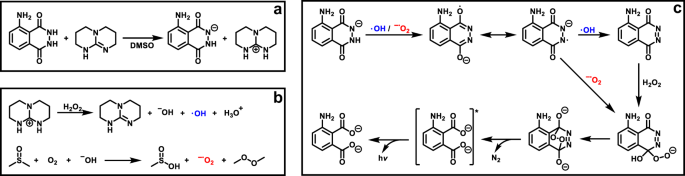
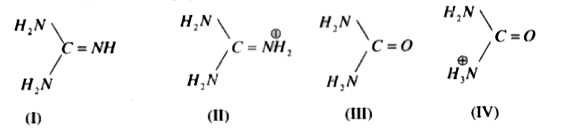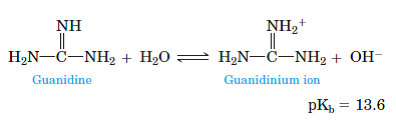A guanidine based bis Schiff base chemosensor for colorimetric detection of Hg(II) and fluorescent detection of Zn(II) ions - ScienceDirect

Syntheses and crystal structures of guanidine hydrochlorides with two Schiff base functions as efficient colorimetric and selective sensors for fluoride

Superbases based on guanidine and the values of pKa of the conjugated... | Download Scientific Diagram
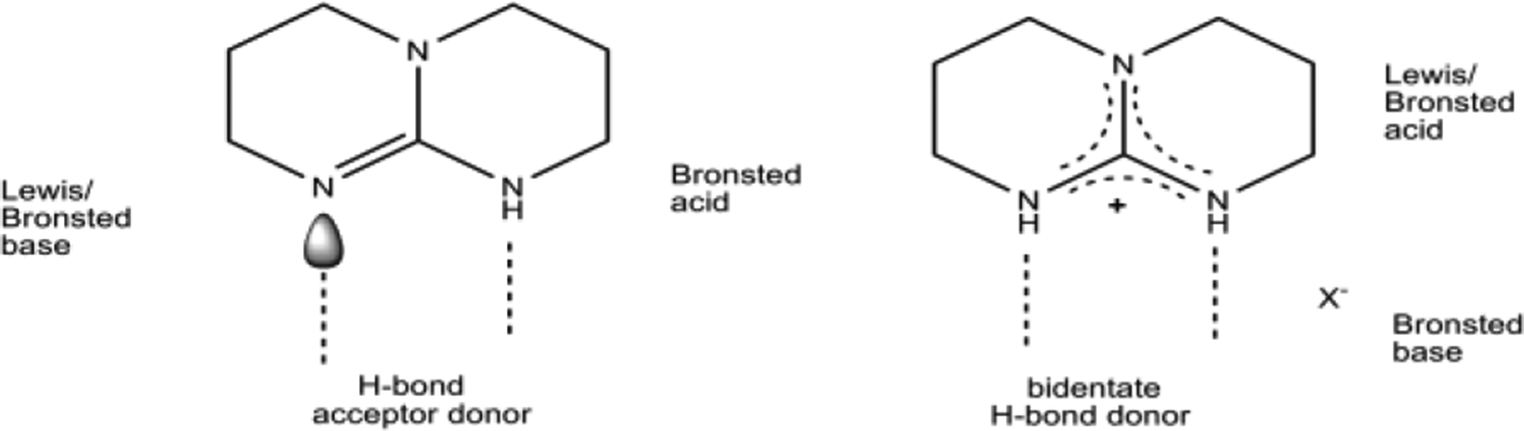
Recent Advances in Guanidine-Based Organocatalysts in Stereoselective Organic Transformation Reactions | IntechOpen

C2‐Symmetric Chiral Pentacyclic Guanidine: A Phase‐Transfer Catalyst for the Asymmetric Alkylation of tert‐Butyl Glycinate Schiff Base - Kita - 2002 - Angewandte Chemie - Wiley Online Library

Figure 1 from Very strong organosuperbases formed by combining imidazole and guanidine bases: synthesis, structure, and basicity. | Semantic Scholar

Sequential Reduction of Nitroalkanes Mediated by CS2 and Amidine/Guanidine Bases: A Controllable Nef Reaction | Organic Letters

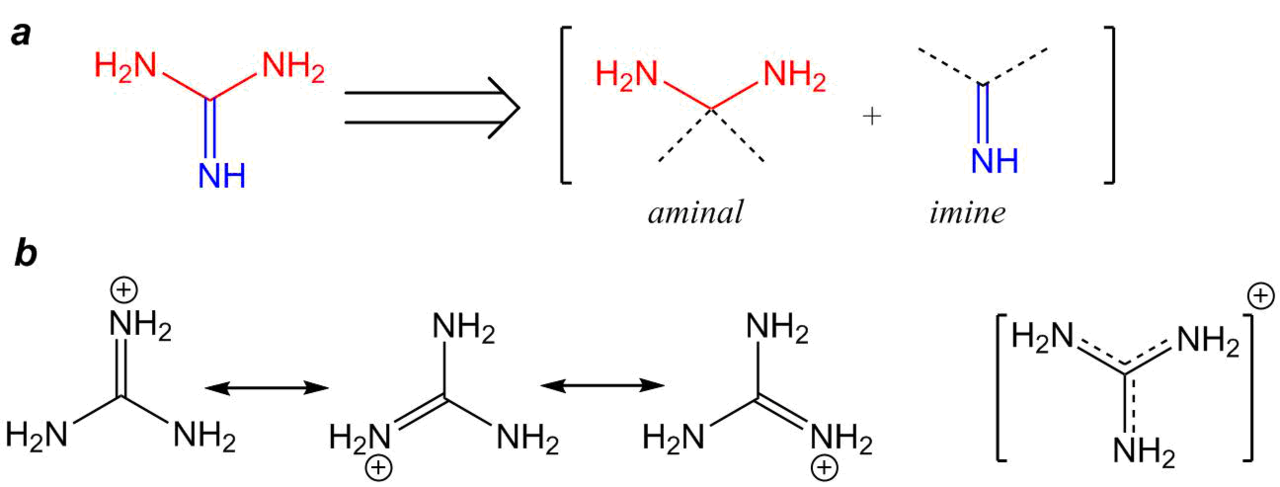
Guanidine and the guanidino group present in arginine are two of the strongest organic bases known. Account for their basicity. | Homework.Study.com
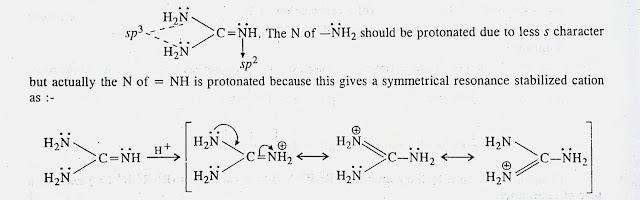
Welcome to Chem Zipper.com......: Give an explanation for the fact that Guanidine NH=C(CH3)2 is a stronger base than most of amines?