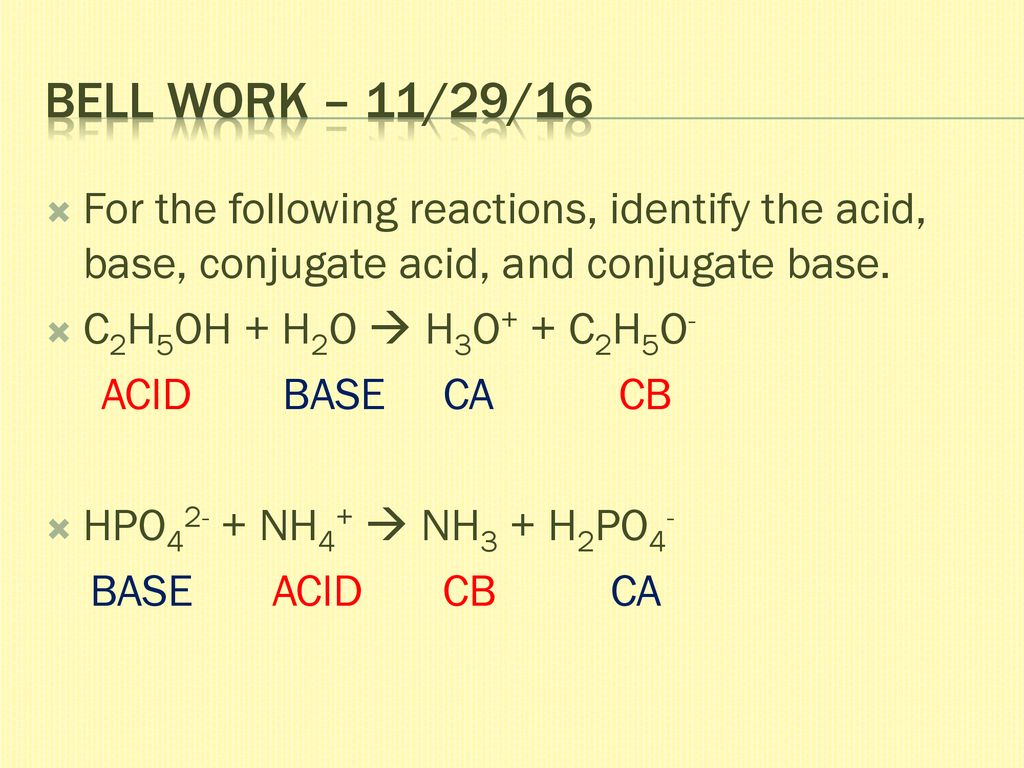
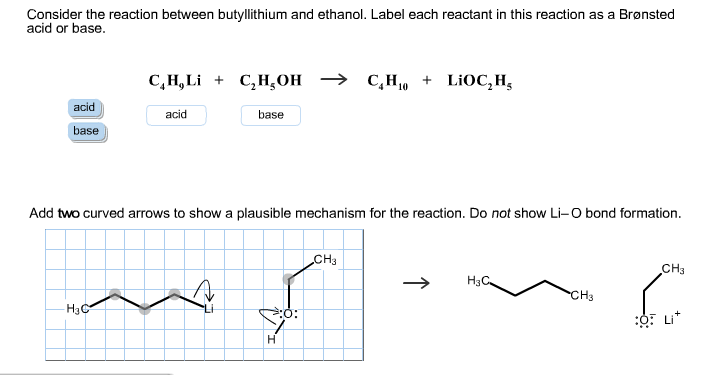
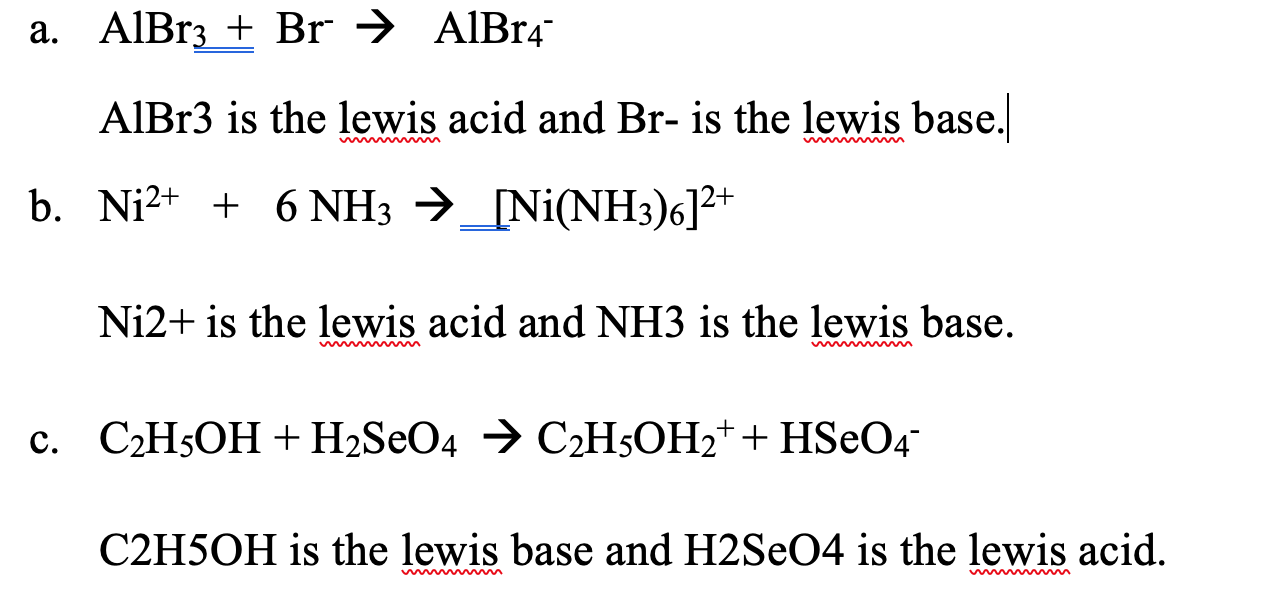Reagents and conditions: (a) CCl3CH(OH)2, NH2OH. HCl, 45min heating;... | Download Scientific Diagram

Growth Pattern, Stability, and Properties of Complexes of C2H5OH and nCO2 (n = 1–5) Molecules: A Theoretical Study | ACS Omega

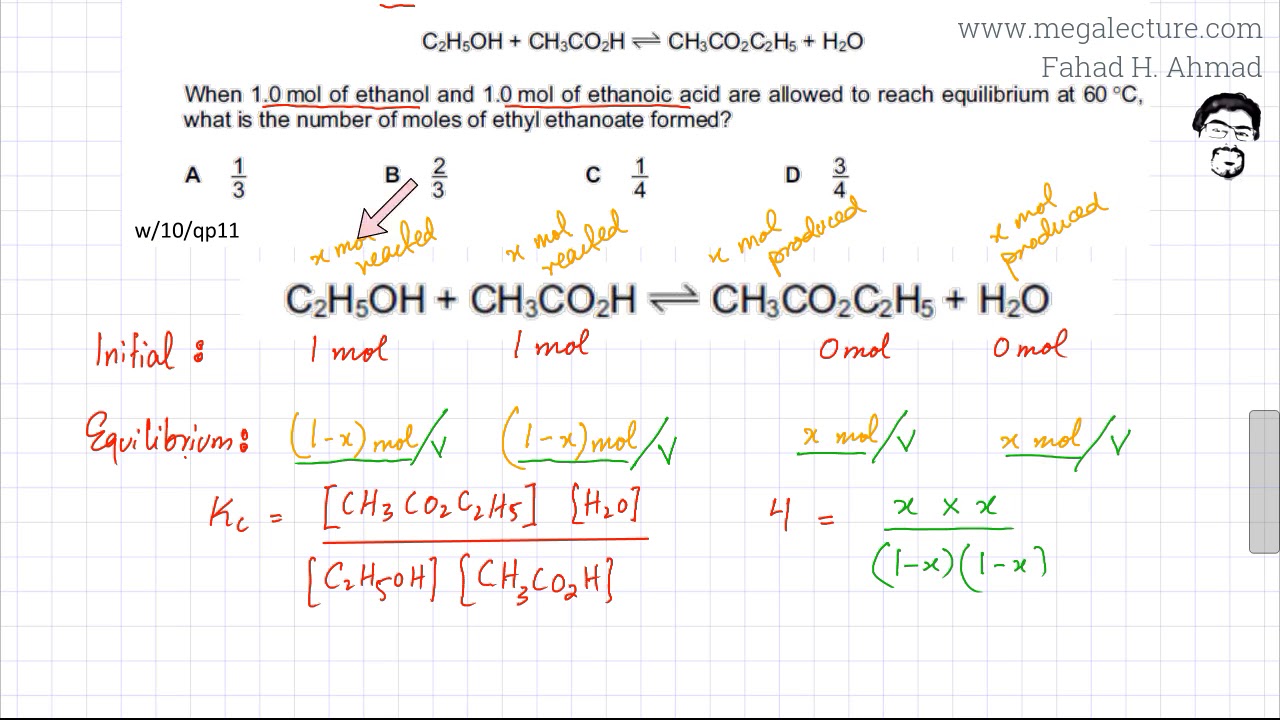
In an experiment starting with 1 mol C(2)H(5)OH, 1 mol CH(3)COOH, and 1 mol of water, the equilibrium mixture mixture of analysis showa that 54.3% of the acid is eaterified. Calculate K(c).

Molecules | Free Full-Text | Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study