Uses of ammonia nitric acid ammonium nitrate salts fertilisers preparation pollution eutrophication gcse igcse KS4 science chemistry O level revision notes revising

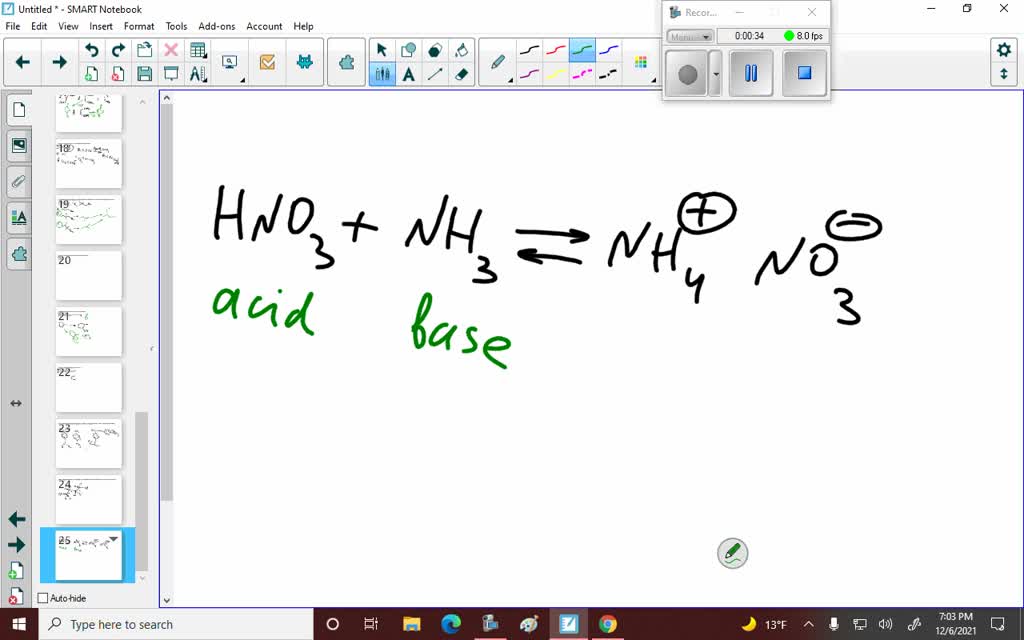
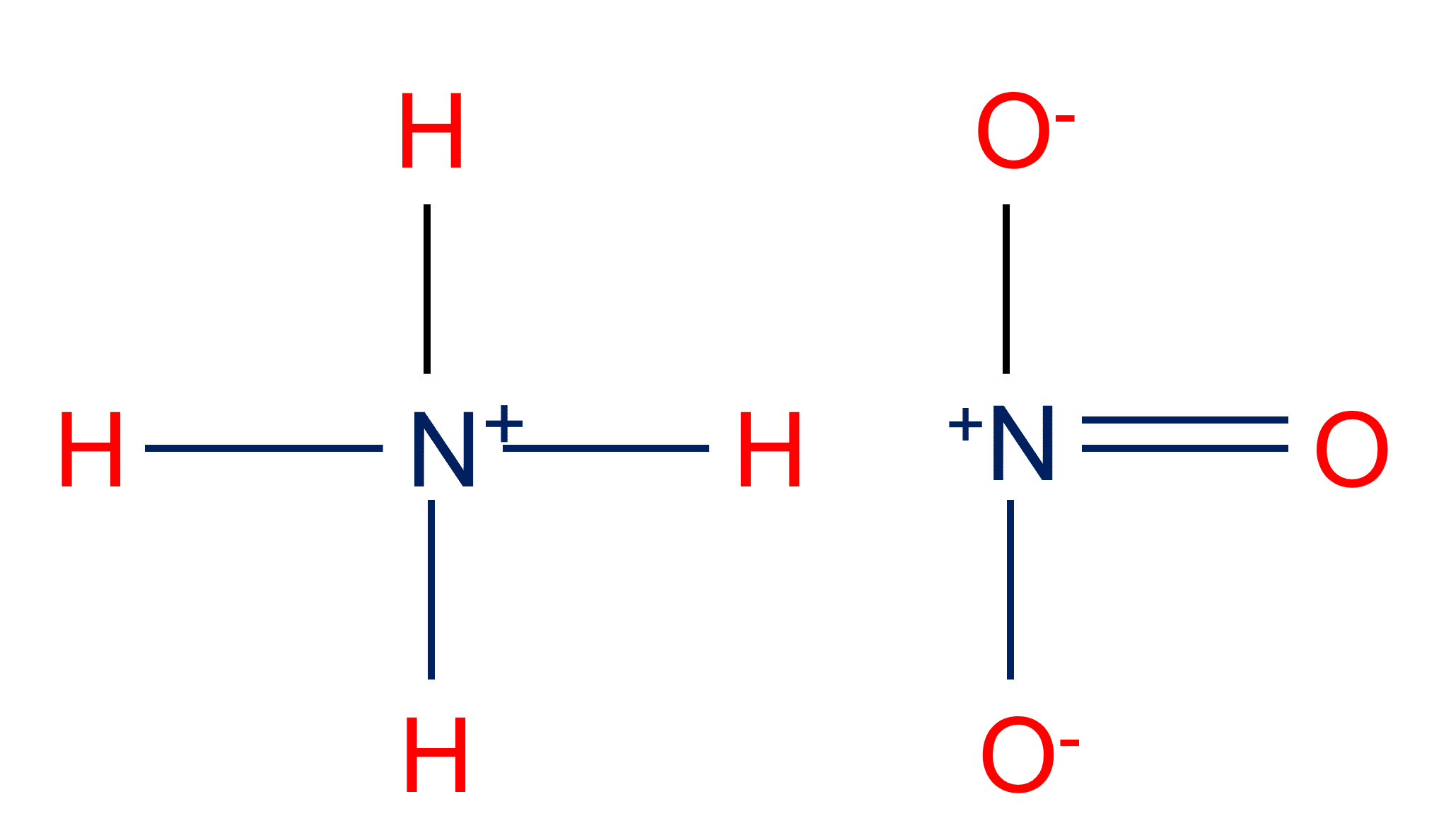
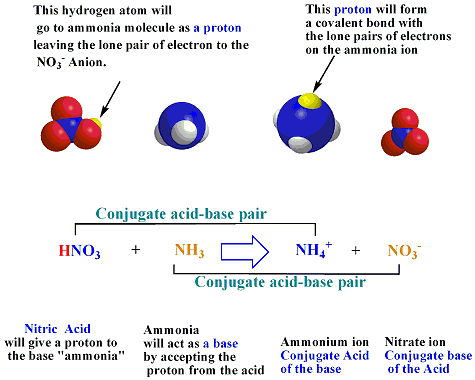
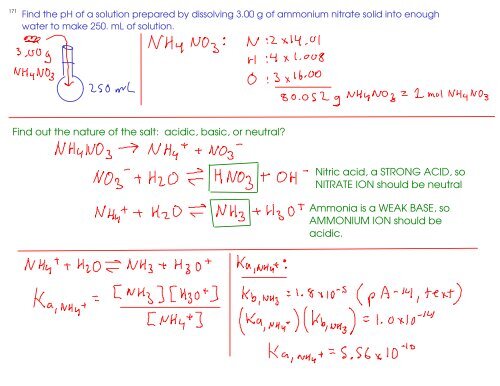
Ammonium nitrate, NH4NO3, is a salt formed from the neutralization of the weak base ammonia with the strong acid nitric acid. Given that the value of Kb for ammonia is 1.8 x

Nature of Salts Green & Damjii – Chapter 8 – Section 18.3 Chang - Chapter 15 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction. - ppt download

Ammonium Nitrate (NH<sub>4</sub>NO<sub>3</sub>) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate

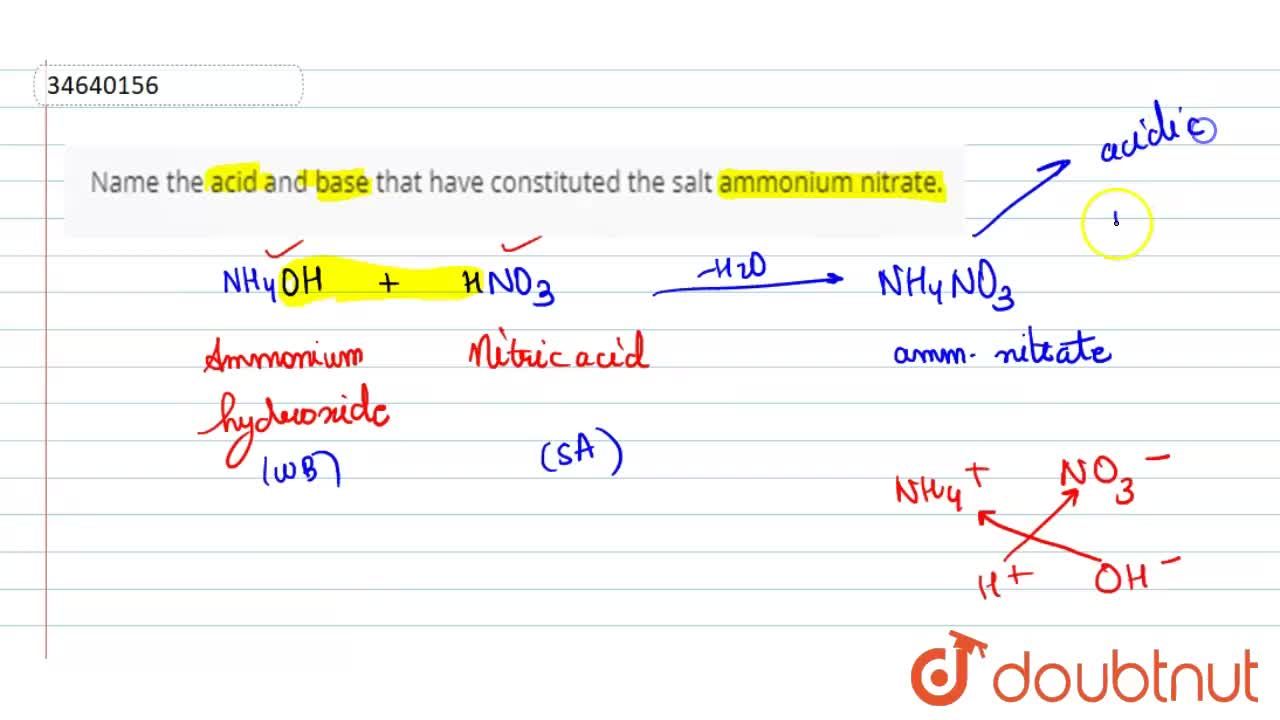
Name the acid and bases needed to form the salt ammonium nitrate give the chemical reaction - Brainly.in

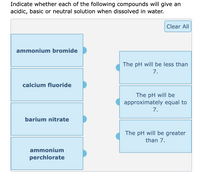
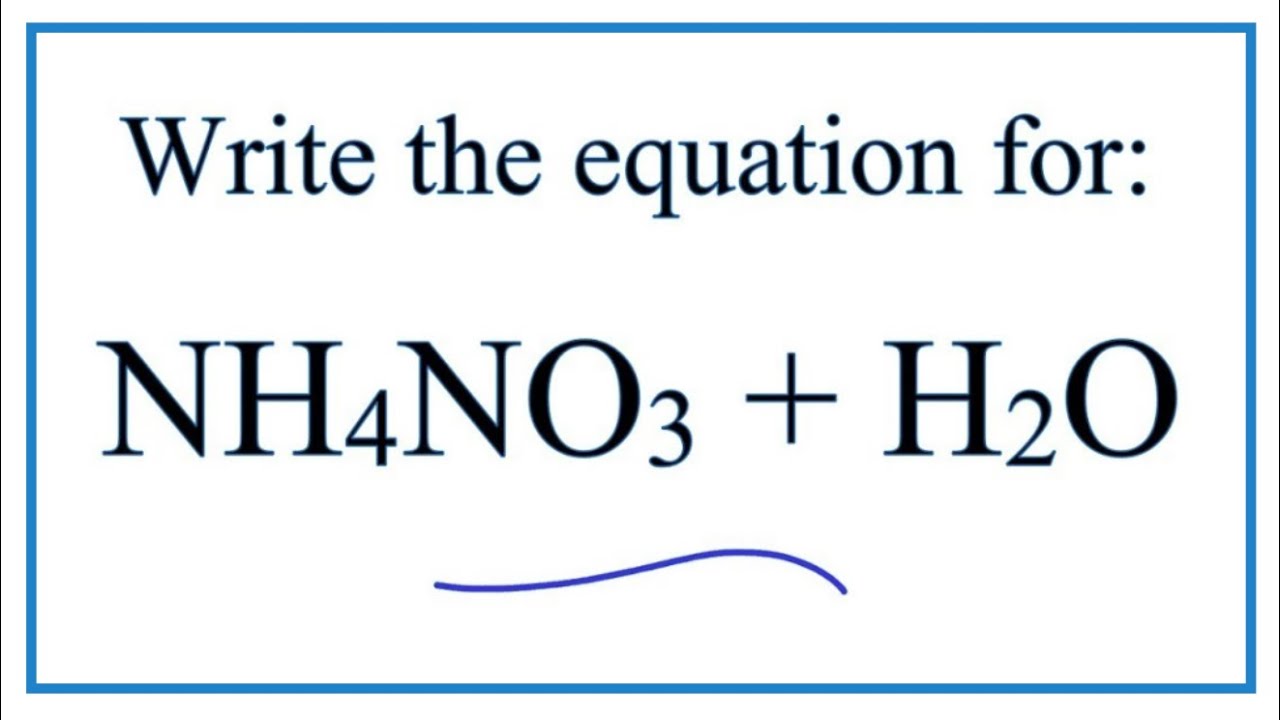
SOLVED: Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium nitrate IS dissolvcd in watcr: (Use HzOt instead of Ht ) HzO() This solution acidic basic
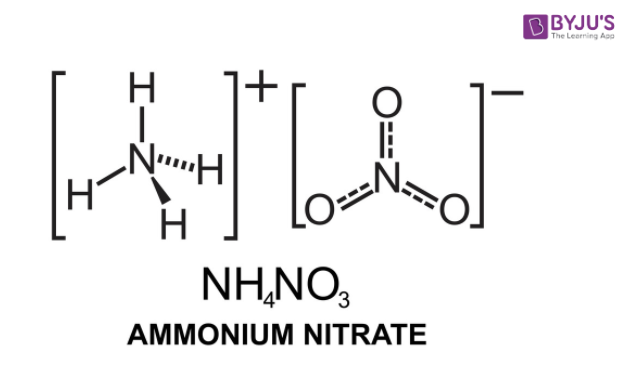
Ammonium Nitrate (NH<sub>4</sub>NO<sub>3</sub>) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate